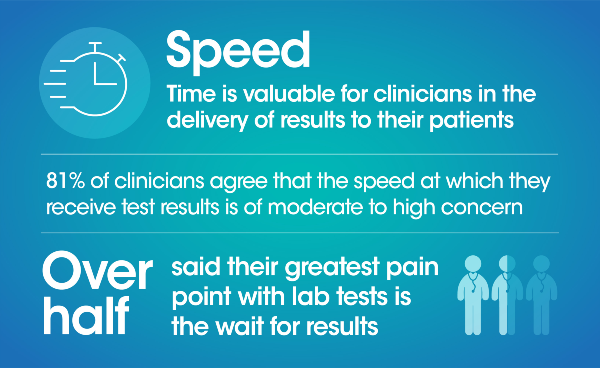
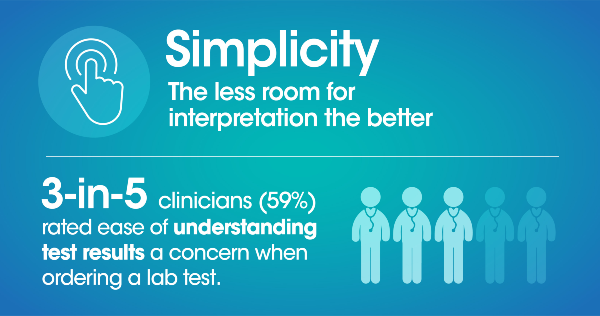
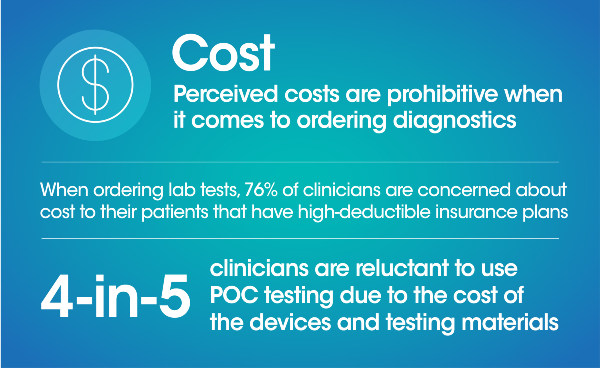
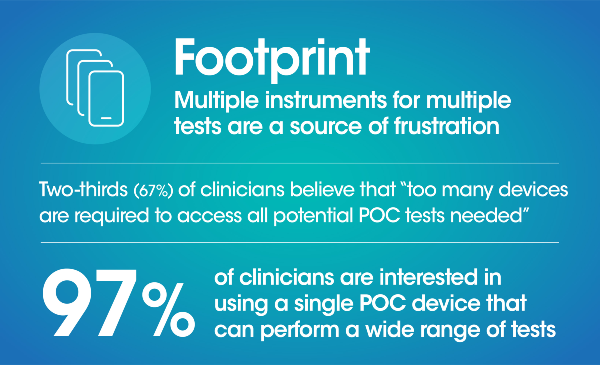
Quality healthcare begins with a diagnosis, and point of care (POC) testing has the ability to make this diagnosis more accessible, more affordable, and more reliable for patients. However, while the pandemic has brought awareness to POC and its ability to provide rapid results there is still uncertainty in its opportunity to persist in the longer term as well as the role it can fill in the diagnostics space.
A blinded third-party survey of 300 US clinicians* was conducted to better understand clinicians’ pain points and perceptions of POC and laboratory diagnostics today in their quest to provide the best in patient care. Through these questions we uncovered the importance of speed, performance, simplicity, cost and footprint for clinicians when evaluating a diagnostic solution.
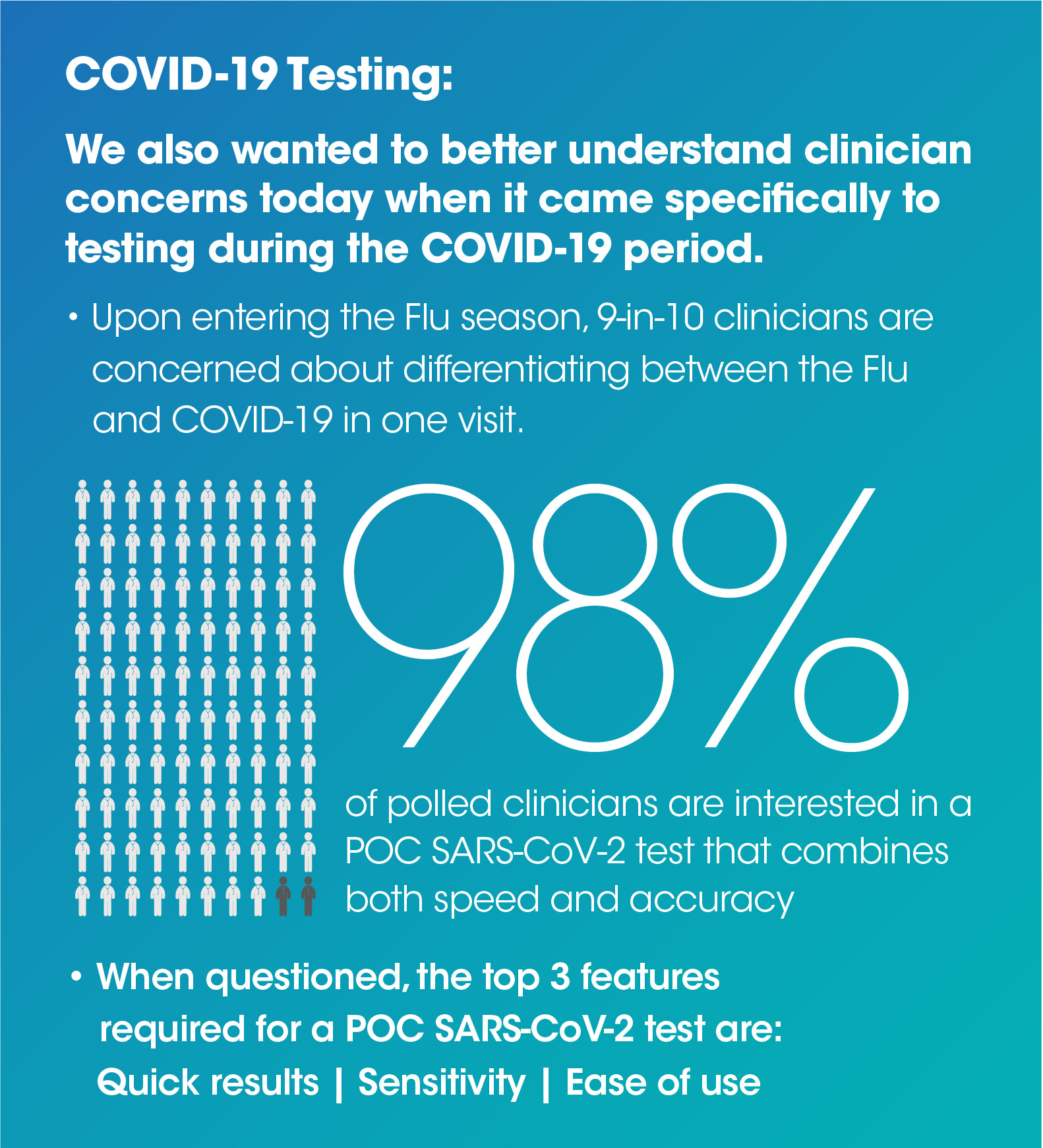
Here's a summary of what we found:
|
Why Compromise?
LumiraDx’s next-gen point of care Platform provides speed without compromising sensitivity at the point of care.
With a growing menu of tests, LumiraDx’s single, easy-to-use, portable instrument and microfluidic technology can aid in diagnosis and allows clinicians to deliver lab comparable results to patients in just minutes.
|
For more information, please visit: www.lumiradx.com/us-en/
About this Survey:
*The third-party online poll of n=300 American clinicians was conducted anonymously between September 10 – 16, 2021. The sample included 150 primary care clinicians and 150 urgent care clinicians, as self-identified. The overall margin of error is ± 5.7%.
The LumiraDx SARS-CoV-2 Ag Test and the LumiraDx SARS-CoV-2 Ab Test have not been cleared or approved by FDA, but have been authorized for emergency use by FDA under an EUA for use by authorized laboratories. The LumiraDx SARS-CoV-2 Ag Test has been authorized only for the detection of SARS-CoV-2 nucleocapsid protein. The LumiraDx SARS-CoV-2 Ab Test has been authorized only for detecting the presence of total antibodies to SARS-CoV-2. They have not been authorized for use to detect any other viruses or pathogens. The emergency use of these Tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic Tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.